 LM Packaging
LM Packaging
Industry Solutions
Medical & Pharmaceutical Labels
-
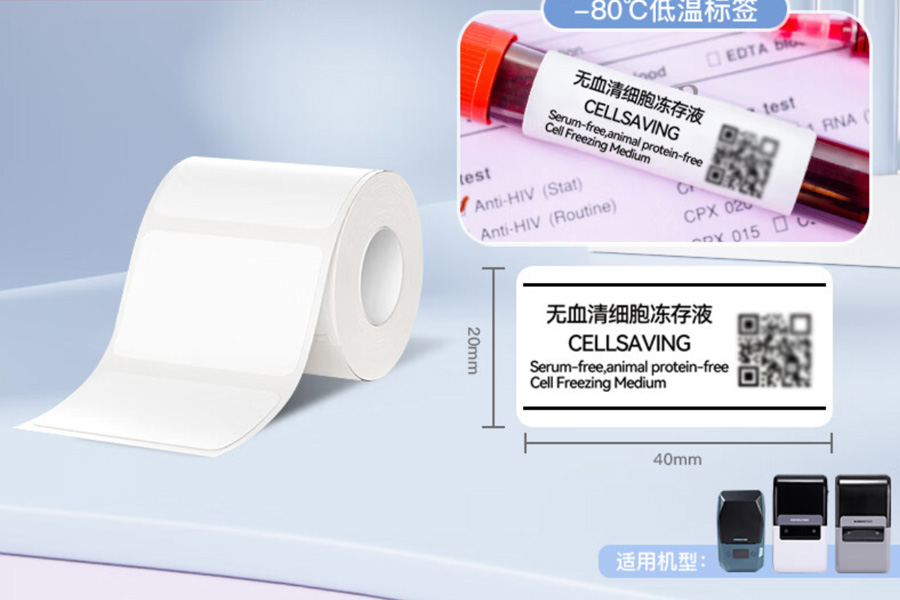
Cold Chain Labels
Cold Chain Labels are high-performance labels specifically designed for the medical and pharmaceutical industry. These labels are ideal for identifying and tracking temperature-sensitive products (such as vaccines, biologics, and pharmaceutical items) during cold chain transportation. Made with high-sensitivity thermal materials and low-temperature adhesive technology, Cold Chain Labels maintain clear print quality and strong adhesion even in extreme cold conditions, such as refrigerated or frozen environments. They meet stringent regulatory requirements while ensuring the integrity and accuracy of critical information throughout transportation. -

Pharmaceutical Labels
Pharmaceutical Labels are an essential component of the medical and pharmaceutical industries. They are used for identifying drug information, providing usage instructions, and tracking the distribution of medications. Our pharmaceutical labels are made from high-quality thermal materials, meeting strict industry standards to ensure clear, durable, and easy-to-read information. -

Lab Sample Labels
Lab Sample Labels are a crucial component of sample management in the medical and pharmaceutical industries. These labels are designed to identify sample information, track sample origins, and record experimental data. Our lab sample labels are made from high-performance thermal materials, specifically designed for the unique demands of laboratory environments, ensuring accuracy, durability, and efficiency in managing samples. -
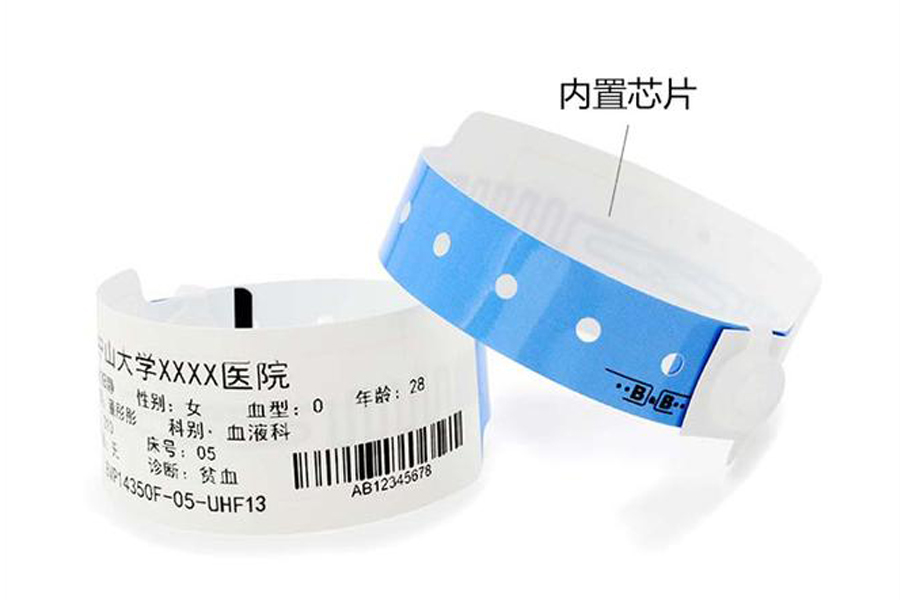
Patient Tracking Labels
Patient Tracking Labels are essential tools in the healthcare industry, widely used in hospitals, clinics, and medical institutions to identify and track patient information, ensuring accurate management from admission to discharge. Our patient tracking labels are made from high-quality thermal materials, providing clear, durable prints and compatibility with various medical systems. These labels help improve patient management efficiency and reduce the risk of operational errors.





